A transformation of fluorouracil (5-FU)
NUC-3373 is specifically designed to overcome the key limitations and pharmacologic challenges that hinder the clinical utility of 5-FU, with the aim of improving 5-FU's efficacy, safety and administration challenges.
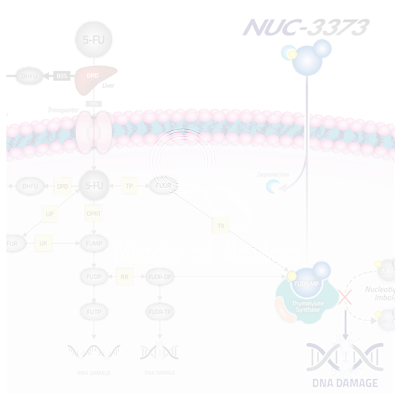
5-FU (and its other forms including capecitabine) is an inactive prodrug and its anti-cancer activity is dependent on its conversion to the active anti-cancer metabolite (FUDR-MP), which binds to and inhibits thymidylate synthase (TS), a critical enzyme in de novo nucleotide synthesis and cell survival. TS is required to convert uridine (specifically dUMP) to thymidine (specifically dTMP), one of the four nucleotides that comprise DNA. The inhibition of TS results in an imbalance in the ratio of dUMP and dTMP, thereby disrupting DNA synthesis and repair, ultimately leading to cancer cell death. However, due to multiple limitations, 5-FU is not efficiently converted to FUDR-MP.
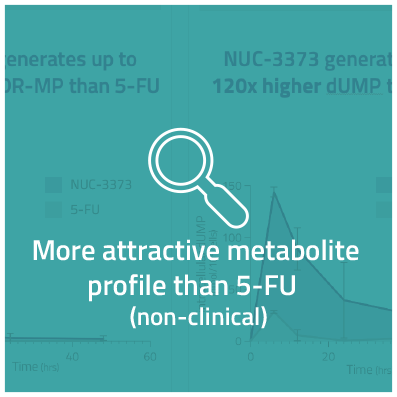
NUC-3373 is a ProTide transformation of 5-FU that generates much higher concentrations of FUDR-MP in patients’ cells. NUC-3373 also has a more convenient administration schedule and does not produce toxic levels of metabolites such as FBAL or FUTP, a toxic metabolite associated with neutropenia, mucositis and diarrhea, which results in an improved safety profile.
NUC-3373 is a more potent and targeted inhibitor of TS.
First introduced in 1957, 5-FU remains a cornerstone of treatment for patients with many types of cancers, including colorectal, breast, gastric, head and neck and pancreatic.
We are developing NUC-3373 as a more effective and safer medicine for patients with colorectal cancer and other solid tumors.
We believe NUC-3373 has the potential to replace 5-FU as the standard of care in the treatment of patients with a wide range of cancers.

Avoids breakdown & minimizes toxic byproducts
More than 85% of administered 5-FU is degraded by the enzyme dihydropyrimidine dehydrogenase (DPD) in the liver. Therefore, most of the drug is broken down before it has an opportunity to enter cancer cells. Levels of DPD have also been found to be elevated in tumors that are resistant to 5-FU. In addition to rendering 5-FU inert, this breakdown results in the generation of a toxic byproduct, FBAL, which has been associated with off-target toxicity including “hand-foot syndrome” which affects 25% to 75% of patients treated with 5-FU and its other forms such as capecitabine. Hand-foot syndrome is a debilitating side effect and commonly causes dose reductions or discontinuation of therapy for patients receiving fluoropyrimidines.
Importantly, 5-FU can also generate a toxic metabolite, FUTP, causing neutropenia, mucositis and GI disturbance.
NUC-3373 avoids breakdown by DPD, and therefore does not generate the toxic byproduct, FBAL, at clinically significant levels. NUC-3373 also does not generate FUTP. We believe this will lead to an improved tolerability profile as compared to 5-FU and capecitabine.

Transporter independent
5-FU relies on specific membrane transporters in order to enter cancer cells. If these transporters are not present or are expressed at inadequate levels, 5-FU’s ability to enter cancer cells will be limited. NUC-3373’s phosphoramidate moiety enables it to enter cells independently of these transporters.

Pre-activated
Once 5-FU enters cells, it must be processed by a series of enzymes to generate the active anti-cancer metabolite, FUDR-MP. There are several key enzymes involved in the conversion of 5-FU to FUDR-MP including orotate phosphoribosyl transferase (OPRT), thymidine phosphorylase (TP) and thymidine kinase (TK). Lower levels of these enzymes in tumor cells are associated with cancer-cell resistance to 5-FU.
Once NUC-3373 enters the cancer cell, the protective phosphoramidate moiety is cleaved, resulting in the release of the active anti-cancer metabolite, FUDR-MP. This bypasses the need for the activating enzymes required by 5-FU and results in significantly higher levels of the active aniti-cancer metabolite, which we believe will lead to improved efficacy compared to 5-FU.

More convenient dosing
5-FU has a short plasma half-life of approximately 10 minutes. As a result, healthcare professionals typically administer 5-FU as a continuous infusion over 46 hours. This causes inconvenience to patients and a significant burden to healthcare systems.
As NUC-3373 is resistant to breakdown by DPD, it has a more favorable pharmacokinetic profile than 5-FU with a more predictable plasma half-life of approximately 10 hours. This enables NUC-3373 to be administered over much shorter time periods (typically two hours). Thus, we believe that NUC-3373 has considerable dosing advantages over 5-FU.
Colorectal cancer
› 1,900,000
New worldwide cases annually
2022
› 155,000
New US cases annually
2022
5-FU-containing regimens are the global standard of care for patients with colorectal cancer. NuCana aims to replace 5-FU with NUC-3373.
Our
Clinical Studies
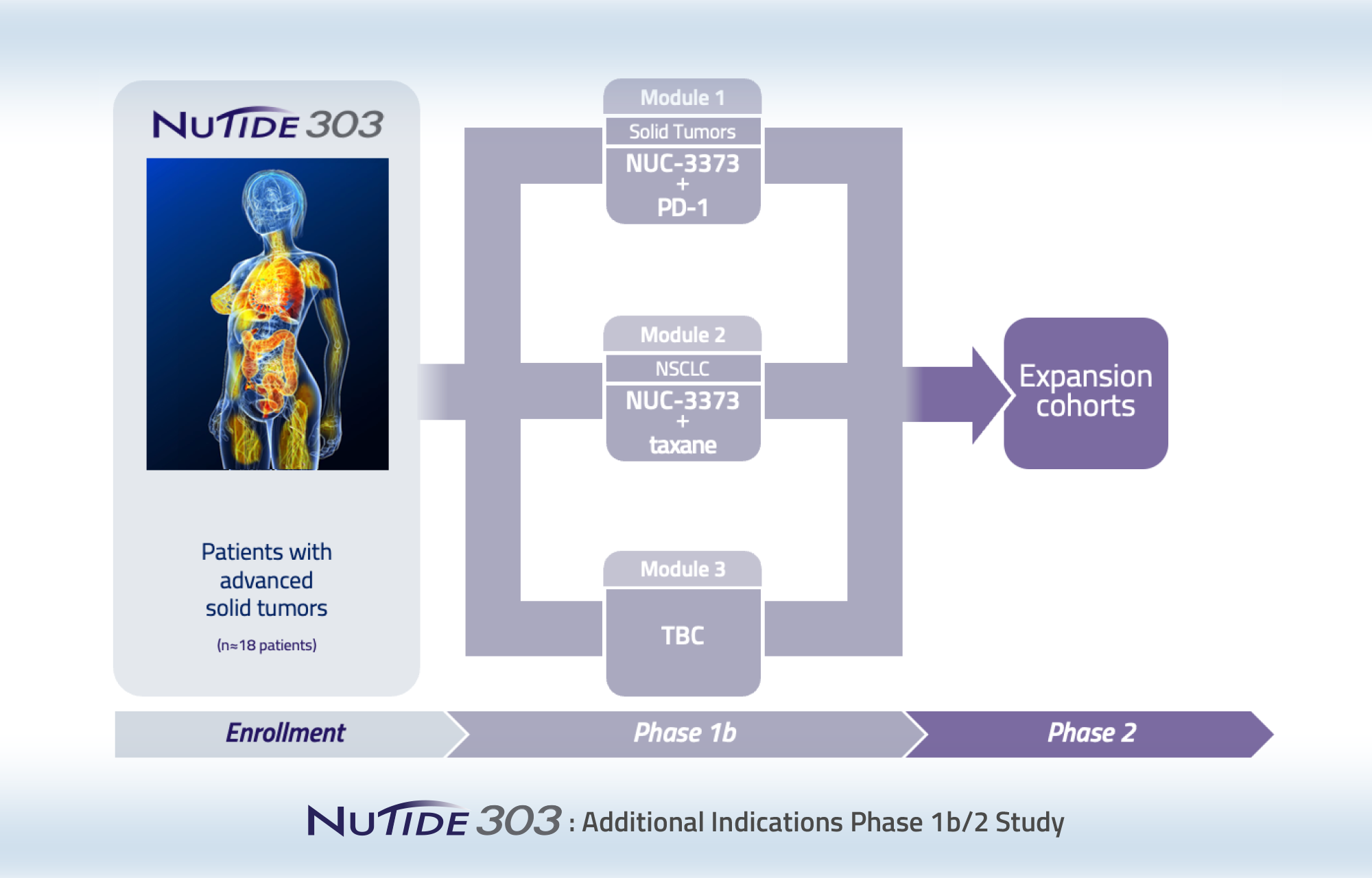
NUC-3373 is currently being evaluated in three ongoing clinical studies: a Phase 1b/2 study (NuTide:302) in combination with leucovorin, irinotecan or oxaliplatin, and bevacizumab in patients with metastatic colorectal cancer; a randomized Phase 2 study (NuTide:323) in combination with leucovorin, irinotecan, and bevacizumab for the second-line treatment of patients with metastatic colorectal cancer; and a Phase 1b/2 modular study (NuTide:303) of NUC-3373 in combination with the PD-1 inhibitor pembrolizumab for patients with advanced solid tumors and in combination with docetaxel for patients with lung cancer.

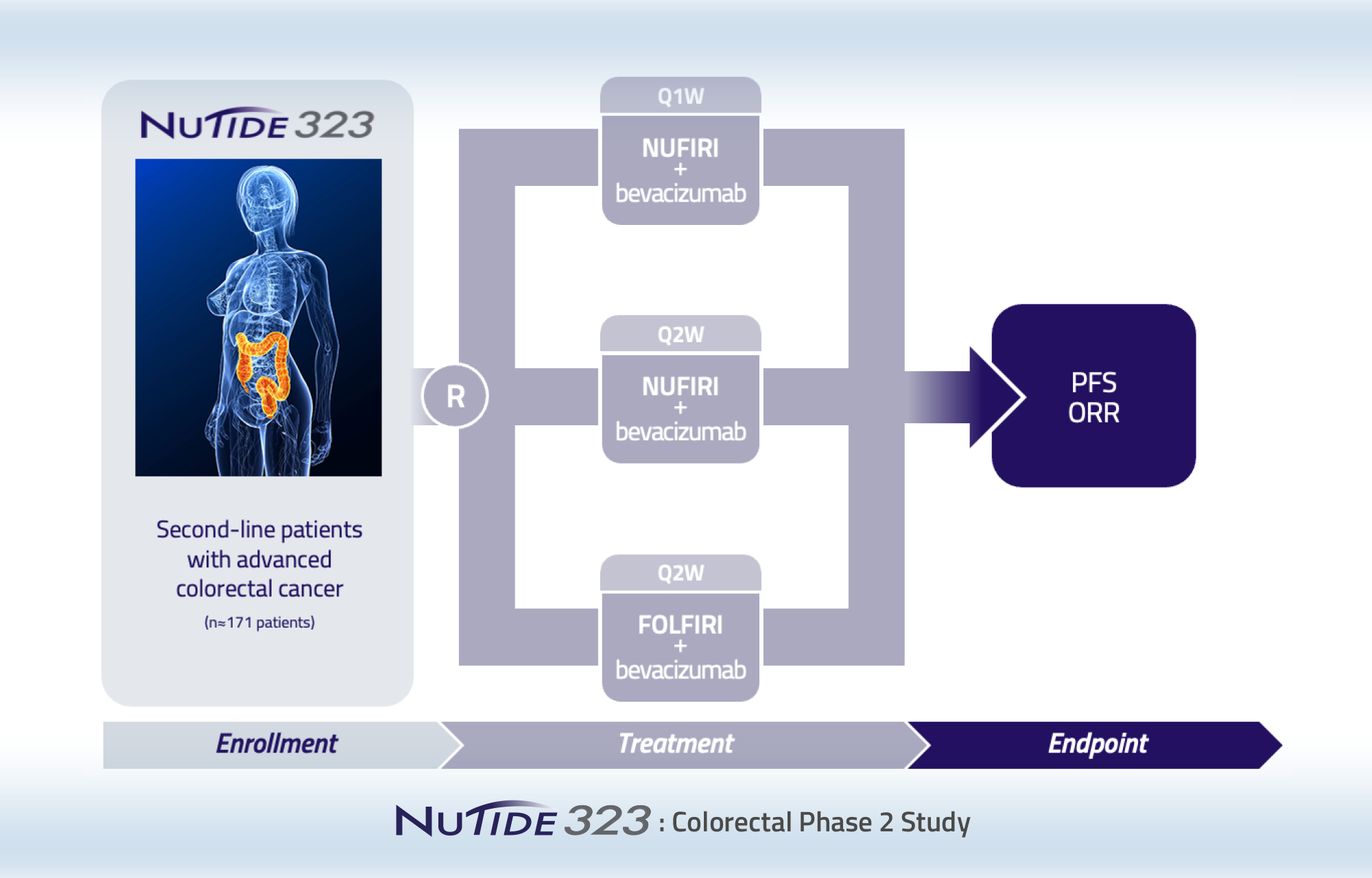
Randomized Phase 2 clinical study of NUC-3373 in combination with leucovorin, irinotecan and bevacizumab (NUFIRI-bev) versus 5 FU in combination with leucovorin, irinotecan and bevacizumab (FOLFIRI-bev) for the second-line treatment of patients with advanced colorectal cancer
This study is evaluating NUC-3373 in combination with leucovorin, irinotecan and bevacizumab in second-line colorectal cancer patients. Two NUC-3373 treatment arms evaluating weekly (Q1W) and alternate weekly (Q2W) administration schedules of NUC-3373 are included as well as one arm with 5-FU, leucovorin, irinotecan (FOLFIRI) and bevacizumab. The study is fully recruited with 182 patients randomized across the three arms.

Phase 1b/2 clinical study to identify additional indications and treatment combinations for further development
This is a modular, multi-arm, parallel-cohort, dose-finding and expansion study of NUC-3373 in combination with other agents for the treatment of patients with different types of advanced solid tumors. There are currently 2 active modules. Module 1 is evaluating NUC-3373 in combination with pembrolizumab in patients with advanced solid tumors who have previously received a PD-(L)1 inhibitor. Module 2 is evaluating NUC-3373 in combination with docetaxel in patients with lung cancer.

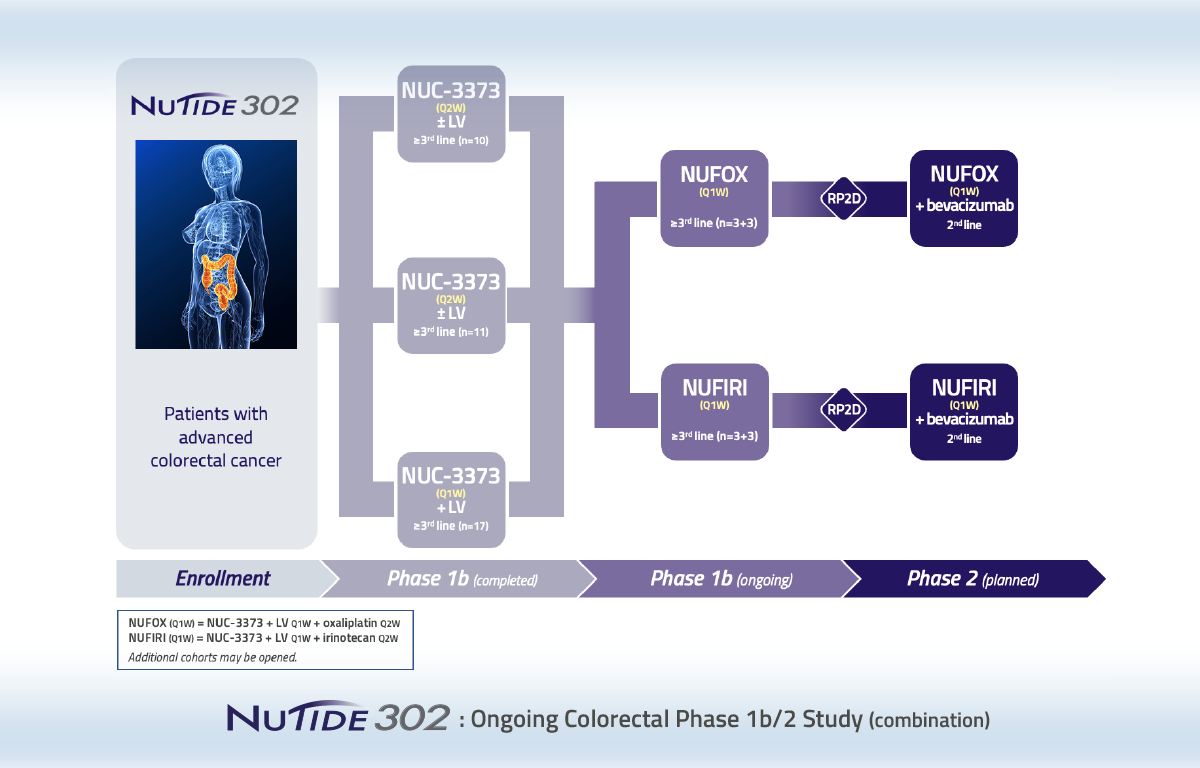
Phase 1b/2 study of NUC-3373 in combination with agents commonly used with 5-FU to treat patients with advanced colorectal cancer
NuTide:302 is a three-part study enrolling patients with advanced colorectal cancer who have previously received fluoropyrimidine-based regimens.
Part 1
Assessed weekly (Q1W) and alternate weekly (Q2W) doses of NUC-3373 with or without leucovorin. The objective of this part of the study was to ascertain whether leucovorin has an impact on the PK and safety profile of NUC-3373.
Part 2
Assessed increasing doses of NUC-3373 plus leucovorin in combination with either oxaliplatin (NUFOX) or irinotecan (NUFIRI). Established the dose of NUC-3373 in NUFIRI and NUFOX combinations to be further examined in Part 3.
Part 3
Established the optimal combinations of NUFIRI and NUFOX with bevacizumab for the second-line treatment of patients with advanced colorectal cancer.
Data from Parts 1 and 2 of NuTide:302 demonstrated a favorable PK and safety profile compared to 5-FU. Data from Part 3 demonstrated a prolonged progression free survival in second-line patients with advanced colorectal cancer compared to their previous line of therapy.
Colorectal Cancer
67 years, female
3 prior lines
-
CAPOX (adjuvant):
for 3 monthsRELAPSED 9 months post adjuvant therapy
-
FOLFIRI:
progressed within 3 months -
Lonsurf:
progressed within 3 months
RAS unknown
Target lesions: 1 (peritoneum)
Colorectal Cancer
69 years, male
2 prior lines
Diagnosed with metastatic disease
-
CAPOX:
progressed within 2 monthstumor increase of 35%
-
FOLFIRI:
progressed within 1.5 months
RAS unknown
Target lesions: 2 (liver)
Colorectal Cancer
52 years, male
5 prior lines
-
FOLFOX (adjuvant):
for 4 monthsRELAPSED 4 months post adjuvant therapy
-
FOLFIRI:
progressed within 6 months -
Irinotecan + panitumumab:
progressed within 6 months -
Irinotecan + panitumumab + telaglenastat:
progressed within 6 months -
Nivolumab + enadenotucirev:
progressed within 3 months
RAS wildtype; BRAF mutant
Target lesions: 3 (2 lung; 1 liver)
* patient missed 6 consecutive doses due to COVID-19 and progressed, but continued on study for a total of 8 months due to clinical benefit
Colorectal Cancer
47 years, male
4 prior lines
-
FOLFOX (adjuvant):
for 5 monthsRELAPSED 8 months post adjuvant therapy
-
FOLFIRI + bevacizumab:
progressed within 18 months -
FOLFIRI + cetuximab:
progressed within 8 months -
Lonsurf:
toxicity within 3 months
RAS wildtype
Target lesions: 5 (2 lymph nodes; 2 peritoneum; 1 liver)
Colorectal Cancer
57 years, male
4 prior lines
-
CAPOX (neoadjuvant/adjuvant):
for 6 monthsRELAPSED 2 months post adjuvant therapy
-
FOLFIRI:
progressed within 3 months -
Lonsurf:
progressed within 2 months -
RXC004 (Wnt inhibitor):
progressed within 1 month
RAS unknown
Target lesions: 3 (lung)
Colorectal Cancer
67 years, female
5 prior lines
-
FOLFOX (adjuvant):
for 5 monthsRELAPSED 2 years post adjuvant therapy
-
FOLFIRI:
for 5 months -
Irinotecan + Lonsurf + bevacizumab:
for 33 months -
CAPOX:
progressed within 1 month -
Regorafenib:
progressed within 2 months
RAS mutant
Target lesions: 2 (1 liver; 1 abdomen)
Graham et al (2020) Ann Oncol 31: Suppl 4 Abstract ID :464P (ESMO poster September 2020)
Coveler et al (2021) J Clin Oncol 39: Suppl 3 Abstract ID: 93 (ASCO GI poster January 2021)

Phase 1 dose-escalation study in patients with advanced solid tumors
Patients
59
Part 1 (n=43) Part 2 (n=16)
Age
59
(range 20-77)
Prior regimens
3
(range 0-11)
Data from this Phase 1 clinical study in patients with advanced solid tumors demonstrated a favorable pharmacokinetic profile of NUC-3373 compared to 5-FU.
Dose proportional increase in AUC (final)
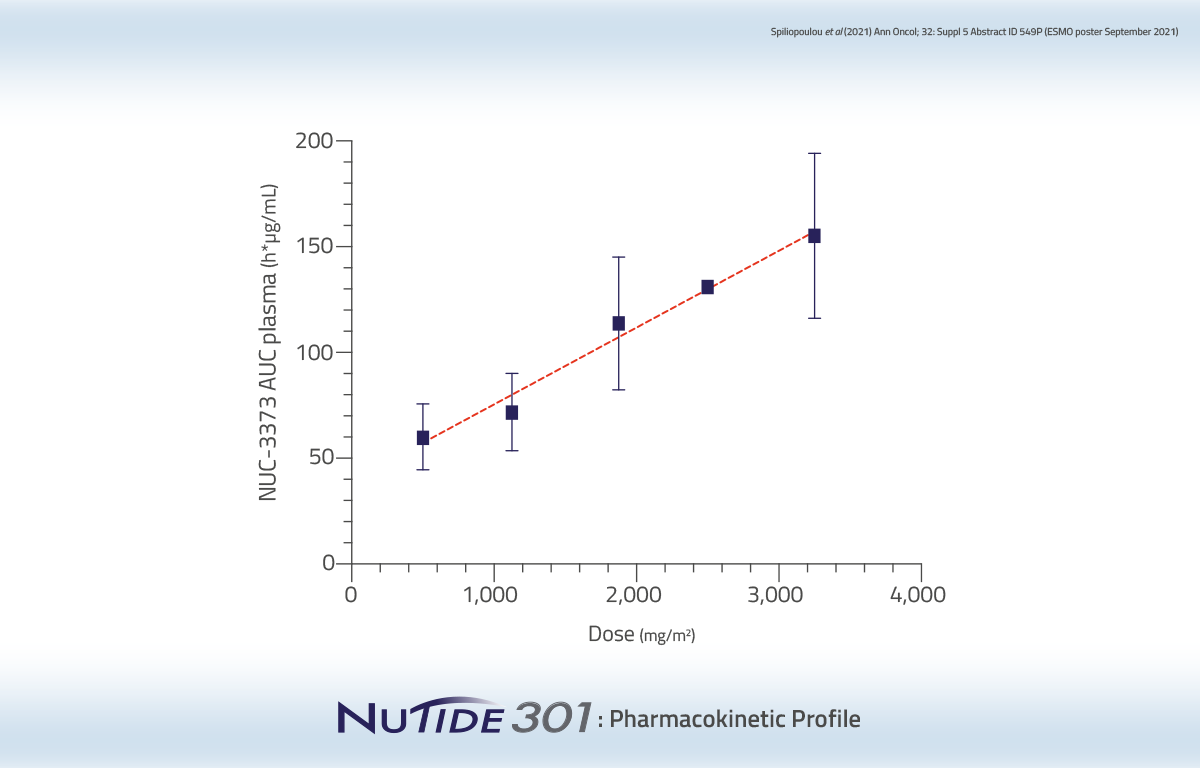
Long plasma half-life compared to 5-FU (6-14 hrs vs 8-14 mins)
• Enables 2-hour infusion vs 46-hour infusion
Anti-cancer activity was observed in patients who had exhausted all standard treatment options.
Metastatic
Colorectal Cancer
70 years, male
6 prior lines
-
5-FU:
based chemoradiotherapy (adjuvant) -
FOLFIRI:
for metastatic disease -
CAPOX:
progressed within 2 months -
FOLFIRI:
progressed within 8 months -
LONSURF:
progressed within 3 months -
Irinotecan:
treatment for 1 month
Metastatic
Basal Cell Carcinoma
55 years, male
2 prior lines
-
Vismodegib:
for 11 months -
Paclitaxel + carboplatin:
for 3 months
Metastatic
Cholangiocarcinoma
60 years, female
1 prior line
-
Gemcitabine + cisplatin:
progressed within 6 months
Blagden et al (2018). Ann Oncol; 29: Suppl 8 Abstract ID: 442TiP (ESMO poster 442TiP, 22nd Oct, 2018)
Data as of Sept 25, 2018
Favorable Safety Profile
- NUC-3373 is well-tolerated (n=59)
- No Grade 4 treatment-related AEs
- Grade 3 treatment-related AEs in 10 patients
- MTD for NUC-3373 monotherapy 2500 mg/m2 Q1W
NUC-3373
Publications
Clinical Presentations
- Poster AACR-NCI-EORTC 2023
(Colorectal Cancer) - Poster AACR-NCI-EORTC 2023
(Solid Tumors/Lung Cancer) - Poster AACR-NCI-EORTC 2023
(Advanced/Metastatic Colorectal Cancer) - Poster ESMO 2022
(Colorectal Cancer) - Poster ESMO 2021
(Colorectal Cancer) - Poster ESMO 2021
(Solid Tumors) - Poster AACR 2021
(Colorectal Cancer) - Poster ASCO GI 2021
(Colorectal Cancer) - Poster ESMO 2020
(Colorectal Cancer) - Poster ASCO GI 2020
(Colorectal Cancer) - Poster ASCO GI 2019
(Colorectal Cancer)
Non-clinical Presentations
- Poster AACR-NCI-EORTC 2022
(Lung Cancer) - Poster AACR-NCI-EORTC 2022
(Lung Cancer) - Poster AACR 2022
(Colorectal Cancer) - Poster AACR 2022
(Colorectal Cancer) - Poster AACR 2021
(Colorectal Cancer) - Poster AACR 2020
(Colorectal Cancer) - Poster AACR-NCI-EORTC 2019
(NUC-3373) - Poster AACR 2019
(Colorectal Cancer)