Story of
Chemotherapy
Discovered in the early 1900’s, chemotherapy refers to the use of specific medicines to eradicate cancer cells from the body or to delay cancer growth. Chemotherapy remains the backbone of treatment for most patients with cancer and it is often used in combination with other agents.
Although significant progress has been made in the development of novel, targeted small molecules and antibodies, these agents have only been successful in certain indications. Furthermore, even if initially effective, most patients will experience disease progression and will therefore receive chemotherapy at some point during their treatment pathway.
While conventional chemotherapy is central to the treatment of many solid and hematological malignancies, its efficacy is limited by cancer cell resistance mechanisms and it is often poorly tolerated. Thus, more effective and safer chemotherapeutic agents will have an important role to play in the treatment of patients with cancer for the foreseeable future.
Limitations of Existing
Nucleoside Analogs

Nucleoside analogs are some of the most widely prescribed chemotherapy agents. They block the replication of cancer cells by providing faulty DNA and RNA building blocks during the cell division process, thus leading to cell death, or apoptosis. Some nucleoside analogs exert their effect by blocking enzymes necessary for the production of these DNA and RNA building blocks.
However, there are major shortcomings that limit the efficacy of nucleoside analogs including:
BREAKDOWN
Susceptible to breakdown and release of toxic byproducts
UPTAKE
Dependent on transporters to enter cancer cells
ACTIVATION
Inefficient and undpredictable generation of anti-cancer metabolites
ADMINISTRATION
Poor PK leads to sub-optimal dosing regimens
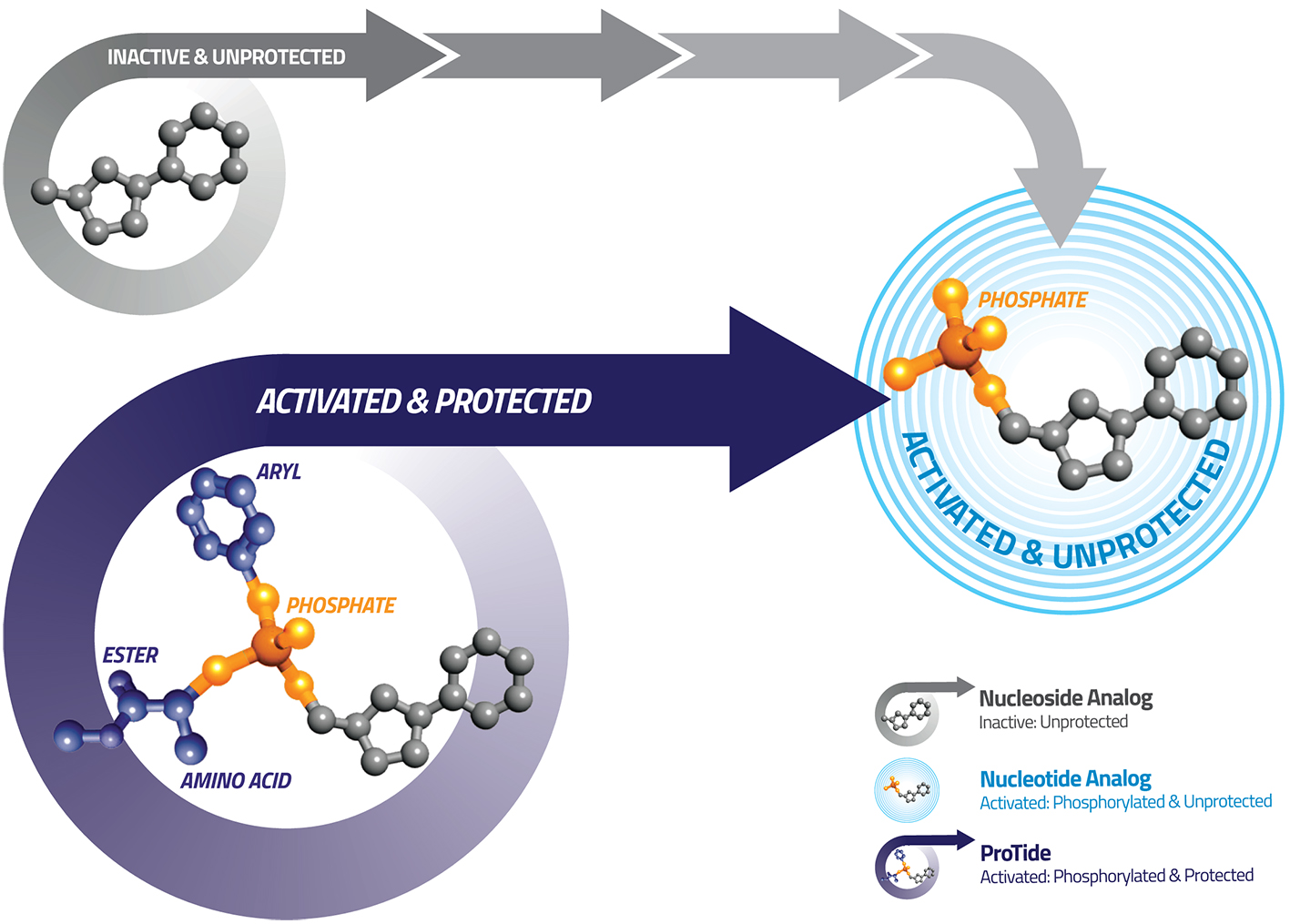
ProTides - A New Horizon for Chemotherapy
Origin of
ProTides
ProTide technology was invented by our late Chief Scientific Officer, Professor Christopher McGuigan at Cardiff University. The unique feature of his discovery was the specific combinations of aryl, ester and amino acid groups (phosphoramidate motifs) that protect the activated nucleotide analog. This phosphoramidate chemistry approach is the key to the ProTide technology.
Every ProTide grouping is distinct, and Professor McGuigan and his team synthesized and tested thousands of compounds in order to identify the optimal phosphoramidate motif for each underlying nucleoside analog.
Professor McGuigan’s work helped lead to the development of several FDA-approved antiviral drugs containing ProTides, including: Gilead's sofosbuvir, (Sovaldi®); and tenofovir alafenamide fumarate (TAF), a ProTide transformation of tenofovir (Viread®); and remdesivir (Veklury®).
The Sovaldi and TAF franchises were the two most successful drug launches in the history of medicine as measured by their first twelve months of revenue post-launch. More recently Gilead’s ProTide remdesivir (Veklury®), was approved for the treatment of patients with COVID-19.
Advantages of
ProTide Technology
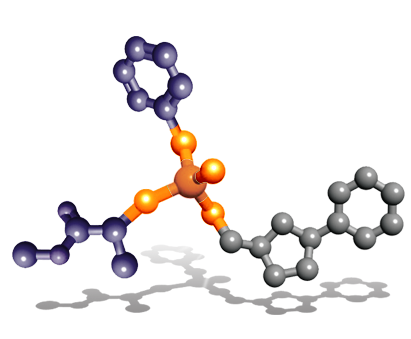

ProTides are rationally designed to overcome the limitations of nucleoside analogs. Our researchers have invested over two decades of work in designing, synthesizing and screening ProTides designed to overcome the key limitations of nucleoside analogs and improve the survival outcomes for patients. Having created thousands of ProTides, we have considerable insight in understanding phosphoramidate chemistry and how our selected ProTides are able to exert their anti-cancer effects.
We are applying ProTide technology to nucleoside analogs currently approved as anti-cancer therapies as well as to novel nucleoside analogs.
NuCana’s ProTides are new chemical entities specifically designed to overcome the key limitations associated with nucleoside analogs:
BREAKDOWN
Resistant to breakdown and minimizing toxic byproducts
UPTAKE
Enter cancer cells independently of transporters
ACTIVATION
Efficient and predictable generation of anti-cancer metabolites
ADMINISTRATION
Improved PK with more convenient dosing regimens
Harnessing the Power of
Phosphoramidate Chemistry
ProTides generate much higher levels of the active anti-cancer metabolites inside tumor cells compared to nucleoside analogs, giving ProTides the potential to be more effective than the current standards of care.
ProTides resist breakdown by deaminase and dehydrogenase enzymes that commonly breakdown nucleoside analogs. This makes ProTides more stable improving their pharmacokinetic profile while also reducing the generation of toxic byproducts that result from the breakdown of nucleoside analogs like 5-FU and capecitabine. Each of our ProTides are distinct anti-cancer agents, with different parent nucleoside analogs, different ProTide structures, different modes of action, and different target indications.
Our pipeline of product candidates in clinical development includes NUC-3373, a new chemical entity derived from the widely used chemotherapy agent 5-FU, and NUC-7738, a new chemical entity derived from a novel nucleoside analog, 3’-deoxyadenosine.
NUC-3373 is currently being evaluated in three ongoing clinical studies: a Phase 1b/2 study (NuTide:302) in combination with leucovorin, irinotecan or oxaliplatin, and bevacizumab in patients with metastatic colorectal cancer; a randomized Phase 2 study (NuTide:323) in combination with leucovorin, irinotecan, and bevacizumab for the second-line treatment of patients with metastatic colorectal cancer; and a Phase 1b/2 modular study (NuTide:303) of NUC-3373 in combination with the PD-1 inhibitor pembrolizumab for patients with advanced solid tumors and in combination with docetaxel for patients with lung cancer.
NUC-7738 is in the Phase 2 part of a Phase 1/2 study which is evaluating NUC-7738 as a monotherapy in patients with advanced solid tumors and in combination with pembrolizumab in patients with melanoma.
The clinical results we have seen to date with NUC-3373 and NUC-7738 have been highly promising and we are extremely encouraged by what has been achieved. This drives us to expedite the development of these potentially important new medicines.