A transformation of 3'-deoxyadenosine (3'-dA)
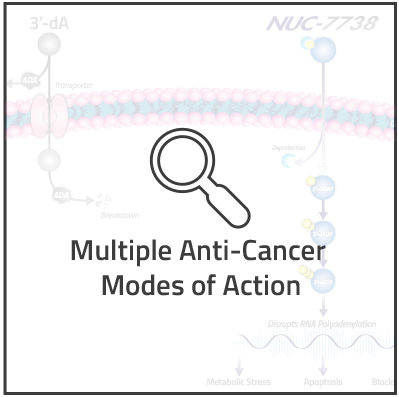
NUC-7738 is a ProTide transformation of 3'-deoxyadenosine (3'-dA), also known as cordycepin. 3’-dA has demonstrated potent anti-cancer activity in non-clinical studies, but has not been successfully developed as a anti-cancer agent due to its rapid breakdown by adenosine deaminase (ADA).
Similar to our other ProTides, NUC-7738 is designed to generate the active anti-cancer metabolite of 3’-dA directly inside cancer cells, thus overcoming 3’-dA's key limitations of breakdown, transportation and activation. The cytotoxic effect of NUC-7738 is largely attributed to the generation of the main active anti-cancer metabolite, 3'-dATP. Primarily, 3'-dATP interferes with RNA polyadenylation, causing changes in the expression of genes involved in metabolism, differentiation and apoptosis, ultimately leading to metabolic stress, cessation of cancer-cell growth and cell death.
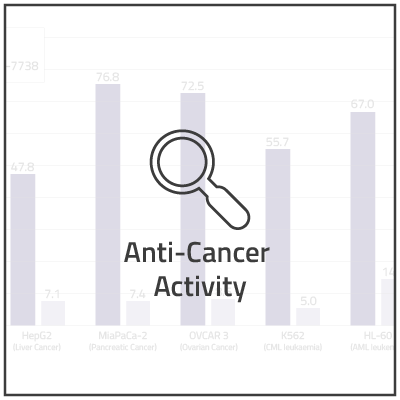
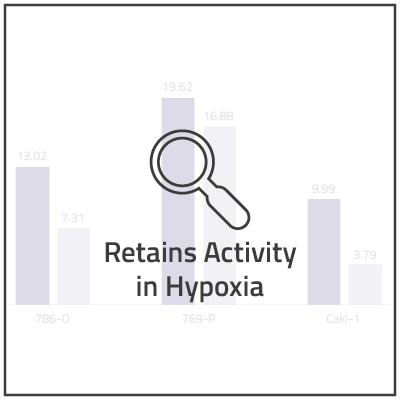
In non-clinical studies, we examined the in vitro cytotoxic activity of NUC-7738 in a range of solid and hematological human cancer cell lines, as compared to 3'-dA. In 16 of the 20 cell lines, NUC-7738 was found to be up to 185-times more potent than 3’-dA.

Resistant to breakdown
3’-dA is rapidly broken down by the enzyme adenosine deaminase, or ADA. This breakdown prevents 3’-dA's generation of the active anti-cancer metabolite. By contrast, NUC-7738 is not a substrate for ADA and is therefore resistant to degradation by this enzyme, thereby also avoiding the generation of breakdown byproducts.

Pre-activated
The rate-limiting activation step of 3’-dA requires the addition of a phosphate group, by the enzyme adenosine kinase, or AK to generate 3’-deoxyadenosine monophosphate (3’-dAMP). This phosphorylation is the rate-limiting step in the generation of the active anti-cancer metabolite 3’-deoxyadenosine triphosphate (3’-dATP). Once inside the cancer cell, NUC-7738 releases 3’-dAMP and as such does not require AK for the rate limiting first phosphorylation step. 3’-dAMP in turn is rapidly converted into 3’-dADP and 3’-dATP.
Our
Clinical Studies
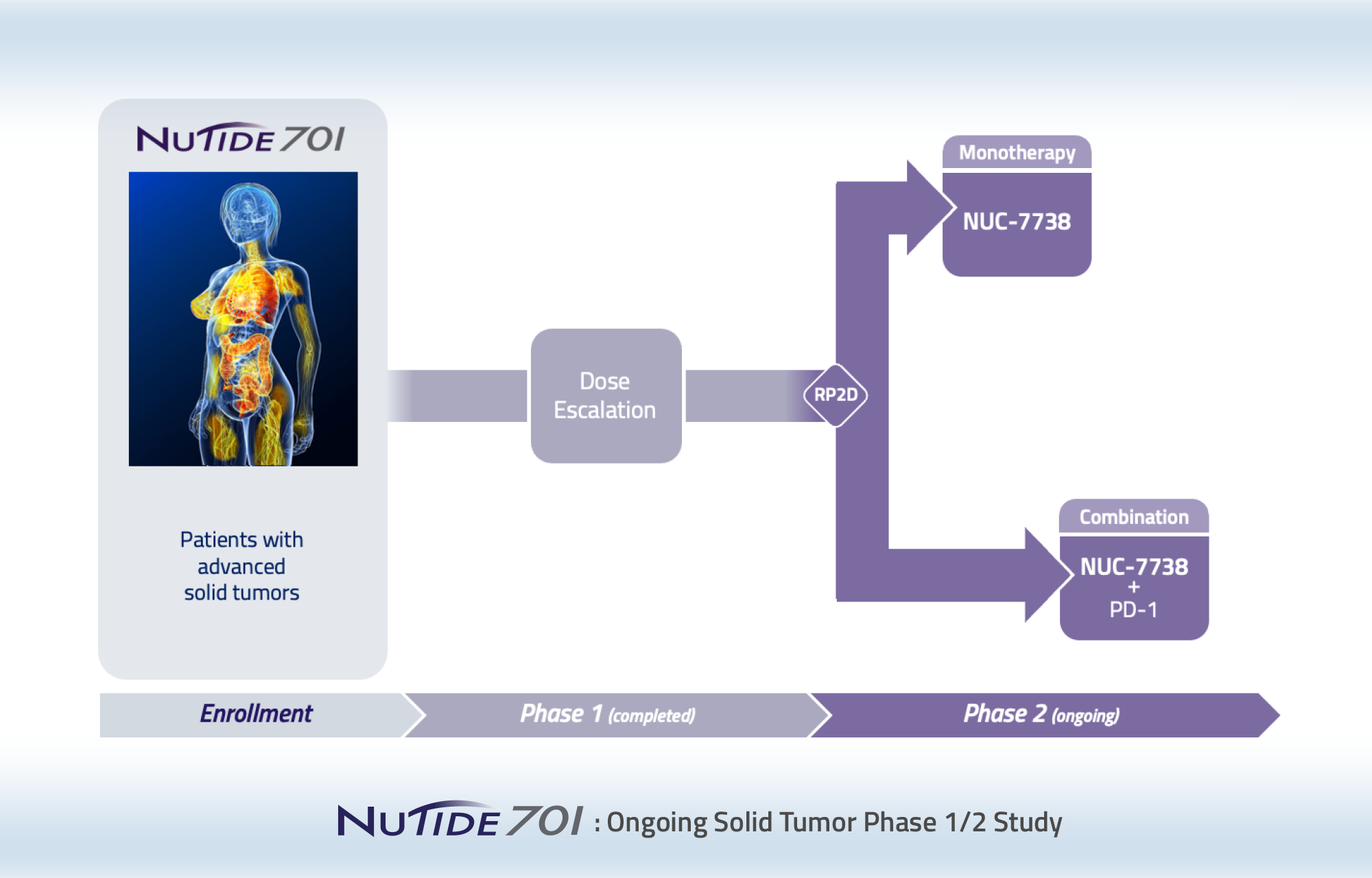
We are developing NUC-7738 for patients with solid tumors or hematological malignancies based on its broad activity observed in non-clinical studies. NUC-7738 is currently being evaluated in a Phase 1/2 study.

Phase 1/2, dose-escalation study of NUC-7738 in patients with advanced solid tumors
This study is currently enrolling patients with advanced solid tumors and has two parts.
Phase 1:
Recommended Phase 2 dose, pharmacokinetics and safety
Phase 2:
Efficacy, pharmacokinetics and safety
Exploratory
Endpoints:
Biomarker evaluation, pharmacodynamics
Metastatic
Melanoma
62 years, female
2 prior lines
-
Nivolumab + ipilimumab:
discontinued within 1 month -
CK7 inhibitor:
progressed within 1 month
Target lesion: 1 (pelvic side wall)
Metastatic
Melanoma
65 years, female
1 prior line
-
Nivolumab + ipilimumab:
discontinued within 1 month
Target lesion: 1 (lung)
Metastatic lung
Adenocarcinoma
65 years, male
2 prior lines
-
Carboplatin + Pemetrexed:
progressed at 6 months -
Docetaxel:
progressed at 4 months
Target lesions: 2 (lung)
* Treatment beyond PD allowed per protocol for patients still receiving benefit.
Blagden et al (2021) Ann Oncol: 32: Suppl 5 Abstract ID 566TiP (ESMO poster September 2021)